Which Atom Has the Greatest Nuclear Charge
Sn-119 Sb-122 Te-128 I-127 3. Na BK OCs Da QUESTIONS Consider the following consecutive ionization energies in kJmol.

Periodic Trend Effective Nuclear Charge Chemistry Video Clutch Prep
Report an issue.

. A If each core electron were totally effective in screen- ing the valence electrons from the full charge of the nu- 714 Arrange the following atoms in order of increasing ef CQ fective nuclear charge experienced by the electrons the n 3 electron shell. The largest known completely stable nucleus ie. In which orbital does an electron in a phosphorus atom experience the greatest effective nuclear charge.
Finally subtract the value of S from Z to find the value of effective nuclear charge Zeff. Which of the following is the largest. Atoms are composed of a nucleus containing positively charged protons and neutral neutrons surrounded by a cloud of negatively charged electrons.
What is the nuclear charge of an atom with a mass of 23 and atomic number of 11. Further Development 119 Electron Arrangements In The First 18 Atoms On The Periodic. 11-578 12 - 1817 13 - 2745 14 - 11557 Which of the elements shown is likely to have these lonization energies.
The nuclear charge of an atom is the total charge of all protons in the nucleus. Ion Radius pm Atomic Number N 3 146 7 O 2 140 8 F 133 9 Na 98 11. A chlorine atom a chlorine ion with a charge of 1- or a bromine atom.
Z eff Z - σ. For example an atom with 12 protons and 12 electrons which is a neutral atom will lose 2 positive charge from the fully occupied first orbital and 8 positive charge from the second. How many neutrons are located in the nucleus of an atom that has 12 protons 12 electrons and an atomic mass of 28 amu.
Which atom has the largest nucleus. As an easy estimation σ is usually close in value to the number of core electrons. Is the right answer 3s then.
N-14 C-12 H-2 He-4. 3p I originally thought 3p since those are the outer electrons but that was marked wrong. The nuclear charge of an atom corresponds to the number of protons in an atom given by the atomic number.
Now put the variables in the formula to know the value of Zeff effective nuclear charge. 11 all atoms in a given sample of an element contain the same number of. Cl-35 Ar-40 K-39 Ca-40 2.
Effective Nuclear Charges for Selected Atoms. Because selenium is directly below sulfur we expect the Se 2 ion to be even larger than S 2 Ionic Radii and Isoelectronic Series. Cl is the largest and has the greatest effective nuclear charge.
Mg or S 3. In this case eqK eq has the largest atomic number and therefore the greatest effective nuclear charge. Nuclear charge is a measure of the effect of the number of protons in the nucleus and their ability to attract the negative electrons in orbits around the nucleus.
O A Na O B. Which of the following has the greatest nuclear charge. QUESTION 8 Identify the atom which has the highest effective nuclear charge Zeff for its valence electrons.
A potassium atom a potassium ion with a charge of 1 or a rubidium atom. Why is it not 3p. Effective Nuclear Charge Chart Table A.
This is the same as the atomic number so for a neon atom it is 10. Zeff 3 17 13. What is the charge of antimony.
The other two electrons in the third orbital do not affect the atoms effective nuclear charge which in this case would be 12 minus 10 or 2. As argon has more number of. Because K has the greatest nuclear charge Z 19 its radius is smallest and S 2 with Z 16 has the largest radius.
The screening constant is the portion of the nuclear charge that is screened from the valence electrons by the core electrons. The sodium cation has the largest effective nuclear charge which results in electrons being held the tightest and therefore Na has the smallest atomic radius. Effective nuclear charge in a Be atom the Is electrons or the 2s electrons.
The nuclear charge of antimony Sb is 51. Which of the following is the largest. Which has the greatest nuclear charge.
Because K has the greatest nuclear charge Z 19 its radius is smallest and S 2 with Z 16 has the largest radius. Sr or O 4. Because chlorine is in the same period as phosphorus and sodium but has the most protons in its shell the most right within the same period it has the greatest effective nuclear charge.
Therefore rubidium has the largest atomic radius whereas helium has the smallest. In each of the above examples Ne F Na an atom has 10 electrons but the effective nuclear charge varies because each has a different atomic number. Al or Si 2.
111 Rutherfords Atom 112 Electromagnetic Radiation 113 Emission Of Energy By Atoms 114 The Energy Levels Of Hydrogen 115 The Bohr Model Of The Atom 116 The Wave Mechanical Model Of The Atom 117 The Hydrogen Orbitals 118 The Wave Mechanical Model. 4 rows Which of the following atoms has the greatest nuclear charge. Stable to alpha beta and gamma decay is lead-208 which contains a total of 208 nucleons 126 neutrons and 82 protons.
Zeff Z- S. The effective nuclear charge Z eff is the number of protons in a nucleus Z minus the screening constant σ. Which has the greatest number of nucleons.
Fluorine has the highest effective nuclear charge. Which element has the smallest atomic radius quizlet. In each pair choose the atom which has the highest first ionization energy based on its position in the Periodic Table.
For example Us the Lithium atom then Z 3 atomic number and S 17. Which elements have the largest effective nuclear charge.
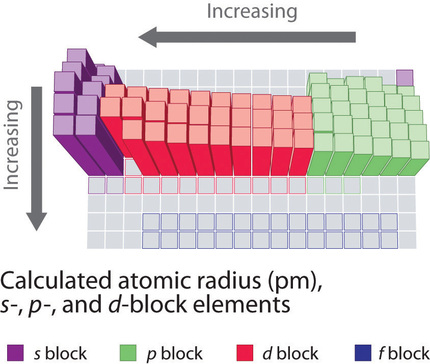
8 6 Periodic Trends In The Size Of Atoms And Effective Nuclear Charge Chemistry Libretexts
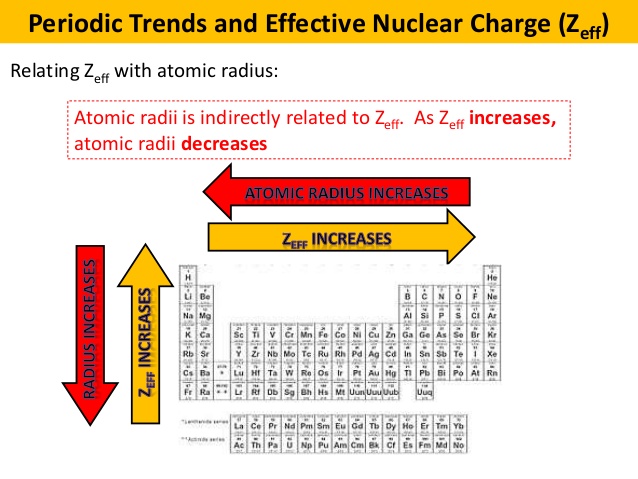
Comments
Post a Comment